An aqueous solution of ethanol MM 4607 gmol has a molality of 1215 m and a density of 1. In organic chemistry one of the most important key points to keep in mind while sketching the electron dot structure is that hydrogen atoms will usually take the terminal positions irrespective of the comparison in the.
Draw The Structure Of Ethanol Molecule Sarthaks Econnect Largest Online Education Community
Both molecular and condensed structural formulas are written on a single line of text but.

. A molecular formula tells only the numbers of atoms of each element in a molecule of the compound. Write the molecular orbital configuration of NO molecule and draw its energy level diagram. The intermolecular forces are both London forces and permanent dipole-dipole attractions.
Which one of these. BCI 3 CH 4 CO 2 NH 3. Based on this structure determine if the molecule is polar or nonpolar.
Draw the structure of the monomer but use CC instead of CC. Predict the shapes of the following molecules on the basis of hybridization. Out of them which one is less important as a resonance structure and why.
Current commercial production of ethanol is based almost exclusively on starch- and sugar-based feedstocks. A condensed structure shows all atoms but it omits the vertical bonds and most or all of the horizontal single bonds. A Draw and label a flowchart of the process and carry out a degree-of-freedom analysis to verify that you can determine all unknown quantities on the chart.
A The atomic number of chlorine is 17. Also calculate its bond order. You may need a periodic table for this.
Where a particular way of drawing a structure is important this will always be pointed out where it arises elsewhere on this site. Always show the detail around the important parts of a molecule. CH 4 has sp 3 hybridization and tetrahedral structure.
Alcohol any of a class of organic compounds characterized by one or more hydroxyl OH groups attached to a carbon atom of an alkyl group hydrocarbon chain. Thats not the same as hydroxide OH- which is ionic in alcohols a hydroxyl group is connected to a carbon atom. Its essential for predicting molecular geometry molecule polarity and reactivity in a compound.
We need to find out the central atom of the given molecule. The Lewis dot structure provides a simple model between the bonds in a molecule and the lone electron pairs. Chapter 3 Alcohols Phenols and Ethers 2 3 Alcohols 4 The Hydroxy OH Functional Group The hydroxyl group OH is found in the alcohol and phenol functional groups.
Hence 4 2 8 valence electrons are used from a total of 32 valence electrons that are available for drawing the lewis structure of. Discover the chemical equation for the oxidation process of ethanol the steps that occur and the product of ethanols oxidation. Negligible quantities of mathrmCO mathrmCO_2 mathrmO_2 text and mathrmN_2 permeate through the membrane.
Catenation is the ability of an atom to form bonds with other atoms of the same element. In electron dot structure the valence shell electrons are represented by cr osses or dots. Before we begin our exploration of stereochemistry and chirality we first need to consider the subject of conformational isomerism which has to do with rotation about single bonds.
A Lewis structure for the molecule benzene C₆H₆ is shown below. For example the important part of an ethene molecule is the carbon-carbon double bond - so write at the very least CH 2 CH 2 and not C 2 H 4. E-12-dichloroethene Boiling point 48oC This molecule is non- polar.
5 PART B 11. We learned in section 21 that single bonds in organic molecules are free to rotate due to the end-to-end sigma nature of their orbital overlapConsider the carbon-oxygen bond in ethanol for. Draw the cis-and trans-forms of 1 3-dimethylcyclobutane.
In phenols OH is connected to a benzene ring. All gas streams are at approximately 1 atm. I Ozone molecule ii Nitrate ion.
A single bond means 2 shared pair electrons. Begin drawing the Lewis dot structure of the molecule. Write its electronic configuration bDraw the electr on dot structur e of chlorine molecule.
In the above structure four single bonds are used for connecting each bromine atom to a carbon central atom. Place bonds between each carbon and fluoride bond using the three one. The polar C-Cl bonds are on the same side of the molecule.
Draw the Lewis Structure. The polar C-Cl bonds are on opposite sides of the molecule. On a piece of paper draw the symbol for the carbon atom and place three fluoride ions and one iodide ion around the carbon.
Alcohols may be considered as organic derivatives of water H2O in which one of the hydrogen atoms has been replaced by an alkyl group typically represented by R in organic structures. For example both ethanol and dimethyl ether have the. Now look at the above structure and count the number of valence electrons we used till now.
As shown in Figure II4 in the United States the ethanol industry is dominated by corn with 915 percent of production capacity from facilities using corn alone and another 79 percent of capacity from facilities using a blend of corn and other grain eg corn and milo. This molecule is polar. 12022021 Create an account.
Draw brackets around the structure with a long bond passing through each one. CH3OH Lewis Structure. Draw the resonating structure of i Ozone molecule ii Nitrate ion.
Draw the resonance structures of cyanate ion. The Lewis Structure of a molecule gives the simplest representation of valence shell electrons around itself. The structure of DNA is dynamic along its length being capable of coiling into tight loops and other shapes.
A molecule of dimethyl ether has 20 valence electrons. Draw the Lewis structure for thiosulfate S₂O₃² with minimized formal changes. Either A T C or G.
It is exhibited by. In all species it is composed of two helical chains bound to each other by hydrogen bonds. Here the valence electrons are represented by small dots and since a single bond consists of two bonding electrons the two dots between two atoms are represented by a line instead which represents a bond between.
BCl 3 has sp 2 hybridization and trigonal planar structure. DNA is a long polymer made from repeating units called nucleotides each of which is usually symbolized by a single letter. One side of the molecule is slightly negative.
Ethanol Molecule Images Stock Photos Vectors Shutterstock
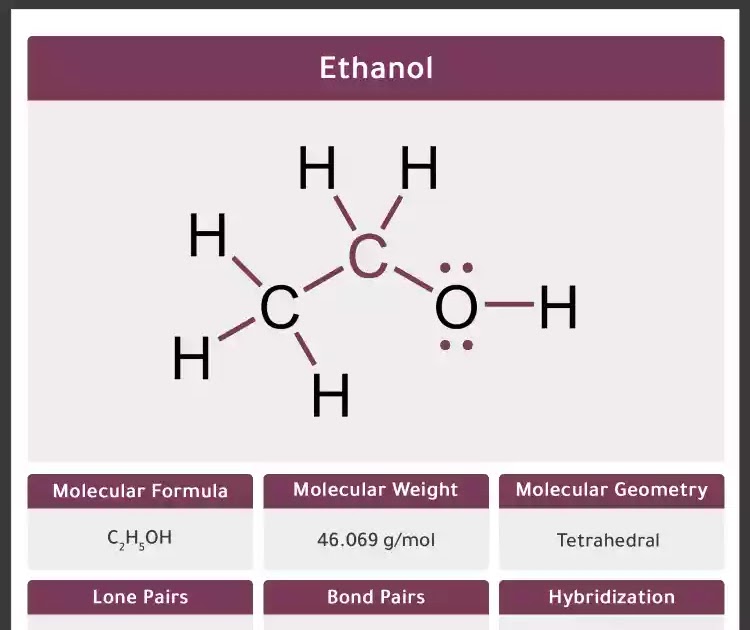
Ethanol Molecular Geometry Hybridization Molecular Weight Molecular Formula Bond Pairs Lone Pairs Lewis Structure Infographic
What Is Alcohol The Alcohol Pharmacology Education Partnership
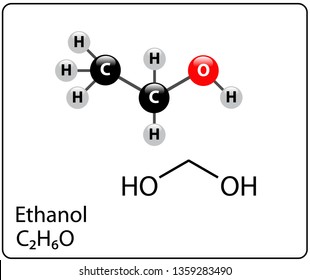
Ethanol Molecule Structure Stock Vector Royalty Free 1359283490 Shutterstock

Ethanol Structural Formula Chemical Structure Alcohol Angle White Png Pngegg

Draw The Structure For Ethanoic Acid Molecule

C2h5oh Lewis Structure Ethanol Youtube
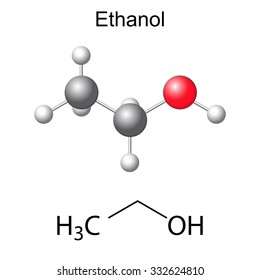
Structural Chemical Formula Model Ethanol Molecule Stock Illustration 332624810 Shutterstock
0 comments
Post a Comment